| Description |
|
Category
|
: Registration
|
|
Renewal Frequency of the Registration
|
: Every Five Years
|
|
Issuing Ministry
|
: The Ministry of Health & Family Welfare
|
|
Incumbent Office
|
Name: Directorate General of Drugs Administration (DGDA)
|
|
|
Address: 105-106, Motijheel Commercial Area, Dhaka-1000
|
|
|
Email: drugs@citech.net
|
|
|
Website: www.dgda.gov.bd
|
Issuance of Registration for Foreign Medicine
|
Required Documents
|
Remarks
|
-
Application by the local nominated representative in Bangladesh
|
Original
|
-
Completed Form DA-1/88
|
Original
|
-
Evaluation fee of Recipe through Treasury Challan
|
Through Bangladesh Bank/Sonali Bank
|
-
Organization’s Profile
|
|
-
Product Profile
|
|
-
Certificate of Pharmaceuticals Products (CPP)/Free sale certificate (FSC)6 Signed by the producing country’s health authority
|
A copy duly attested by the Bangladesh Embassy of that concern county
|
-
In case of medicine for human being:
-
FSC/CPP of Country of Origin (if Australia, France, Germany, Switzerland, Japan, UK, USA )
|
|
-
In case of veterinary Medicine Registration :
-
CPP of country of origin (Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Japan, The Netherlands, Norway, Singapore, Sweden, Switzerland, UK, USA, Russia, Poland, Spain and South Korea)
-
FSC/CPP from one of the 24 countries (if the country is none of the above mentioned then Country of Origin)
|
|
-
Packet sample in English/Bengali and Brochure
|
|
|
Process Steps
|
|
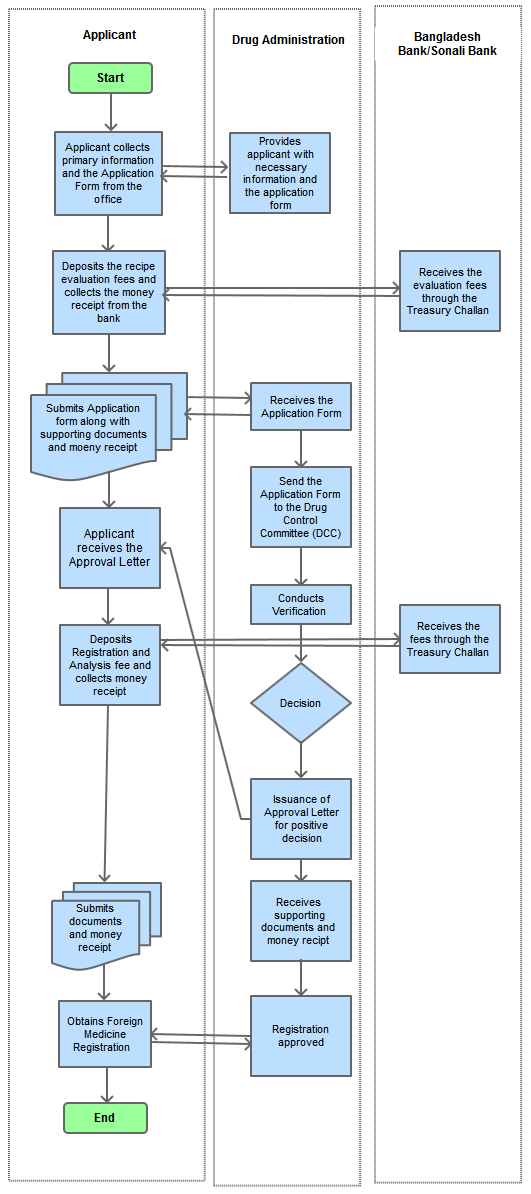
Step 1
|
Collects information form the Drug Administration Office
|
|
Step 2
|
Deposits by Treasury Challan and collects the Money Receipt from the Bank
|
|
Step 3
|
Submits the filled- in Application From along with supporting documents, Treasury Challan/Money Receipt
|
|
Step 4
|
Drug Control Committee (DCC) evaluates the Application and Recipe
|
|
Step 5
|
DCC decides whether to provide with the Registration for Foreign Medicine
|
|
Step 6
|
Issues an approval letter if the decision is positive
|
|
Step 7
|
Applicant deposits and submits the Registration Fee, Analysis Fee, CPP/FSC and Packet of the Product (according to the issued letter)
|
|
Step 8
|
Approval of Registration
|
Process Map:
Renewal of registration for Foreign Medicine
|
Required Documents
|
Remarks
|
-
Certificate of Pharmaceuticals Products (CPP)/Free Sale Certificate (FSC) signed by the producing country’s Health Authority
|
|
-
Packet Sample in English/Bengali and Brochure
|
Original
|
|
Process Steps
|
-
Deposits the renewal and other fees and collects the Money receipt from the bank
|
-
Submits Application along with the supporting documents and Money Receipt
|
-
Completion of the renewal of the Registration
|
|
General Information
|
|
Legal Basis of the Registration
|
The Drug Act – 1940
Drug Control Ordinance – 1982, Section 5
|
|
The nature of the Registration
|
Sector specific (Pharmaceutics)
|
|
The purpose of the Registration
|
Operational
|
|
Territorial Scope of the Registration
|
National
|
|
Eligibility Criteria to obtain the registration
|
Trade License/Business Entity
|
|
Information availability
|
-
Written Procedures are easily accessible by the public from Directorate General of Drug Administration (DGDA)
-
Forms are available online
-
There is no Help Desk in the office
|
|
|---|